This article discusses different types of buffers in our bodies. to diagnose pancreatic diseases. Here, learn about the mechanism of action and more.
Keywords: pH| buffers| protein buffers | hemoglobin| phosphate | carbonic acid-bicarbonate | acidosis | Alkalemia|alkalosis
Table of contents
| 1. | Introduction |
| 2. | Types |
| 3. | Mechanism of action |
| 4. | Abnormal conditions |
About’ totalphysiology.com.’ and learn-and-fly. co. in.’
This article is part of my mission to provide trustworthy recent health information to support the general public, patients, and professionals globally. Here you will find human Physiology, Anatomy, and health-related topics.
Acid-base disturbance
The acidity or alkalinity of any solution is indicated on the ph scale.
The ph scale is from ‘0’ [strongly acidic]to 14[strongly alkaline].
The proper pH of the human body is maintained at a very constant level that is between 7.35 to 7.45. Therefore, a narrow range of pH is essential for normal physiological functions.
A chemical system that resists change in the fluid Ph is a buffer system.
The body buffers, kidneys, and respiratory system mainly maintain body pH.
The primary buffers in the body fluids are:
1. Bicarbonate buffer system
2. Hemoglobin buffer systems
3. Protein buffer systems, and
4. Phosphate buffer systems.
The respiratory system rapidly(minutes) adjusts the blood pH, while the renal system adjusts blood pH slowly (hours to days)by excretion of H+ ions and conservation of bicarbonate.
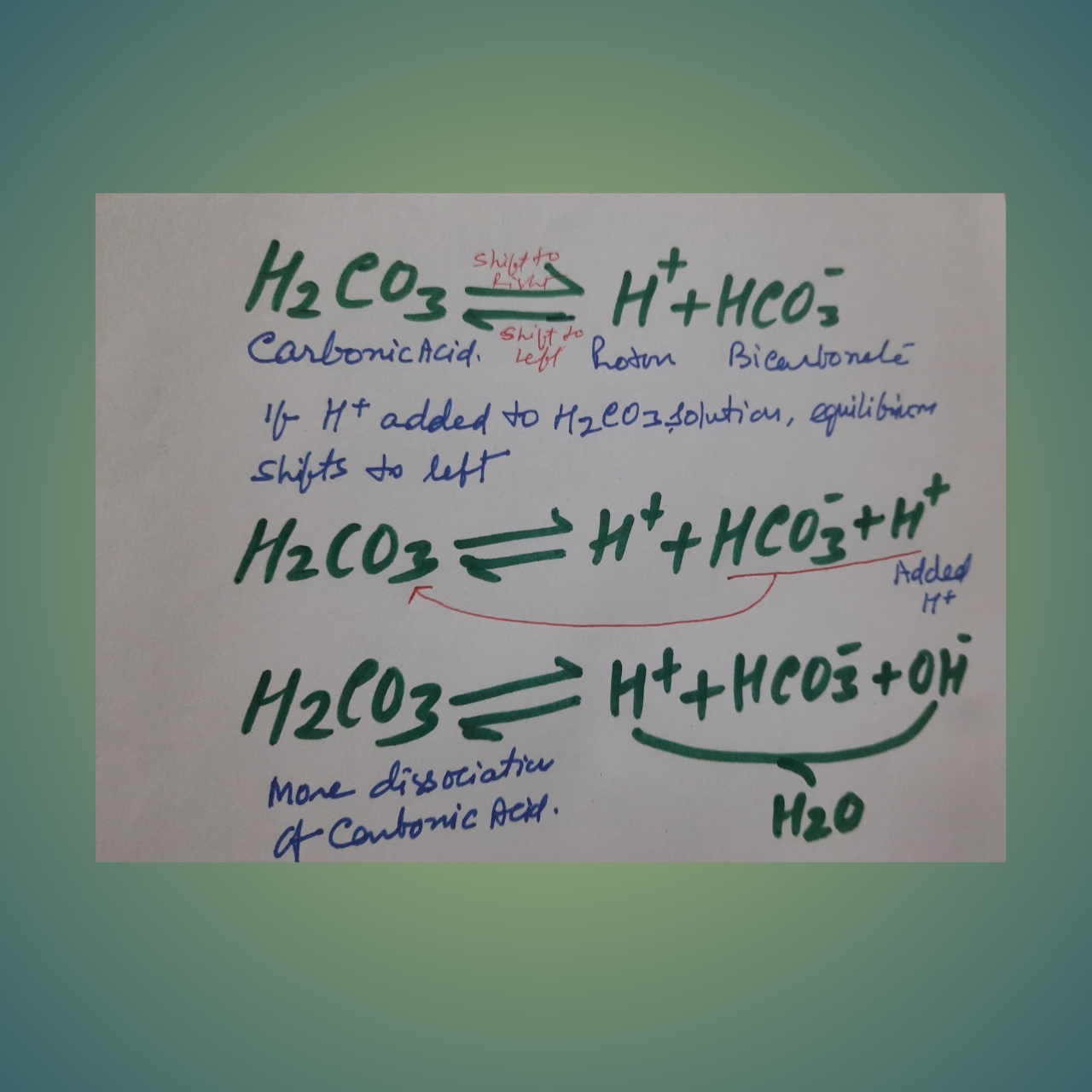
Bicarbonate buffer system= carbonic acid-bicarbonate system: In this system, Carbonic acid (a weak acid), along with its conjugate base, is present. Carbonic acid is a weak acid and dissociates into hydrogen and bicarbonate ions.
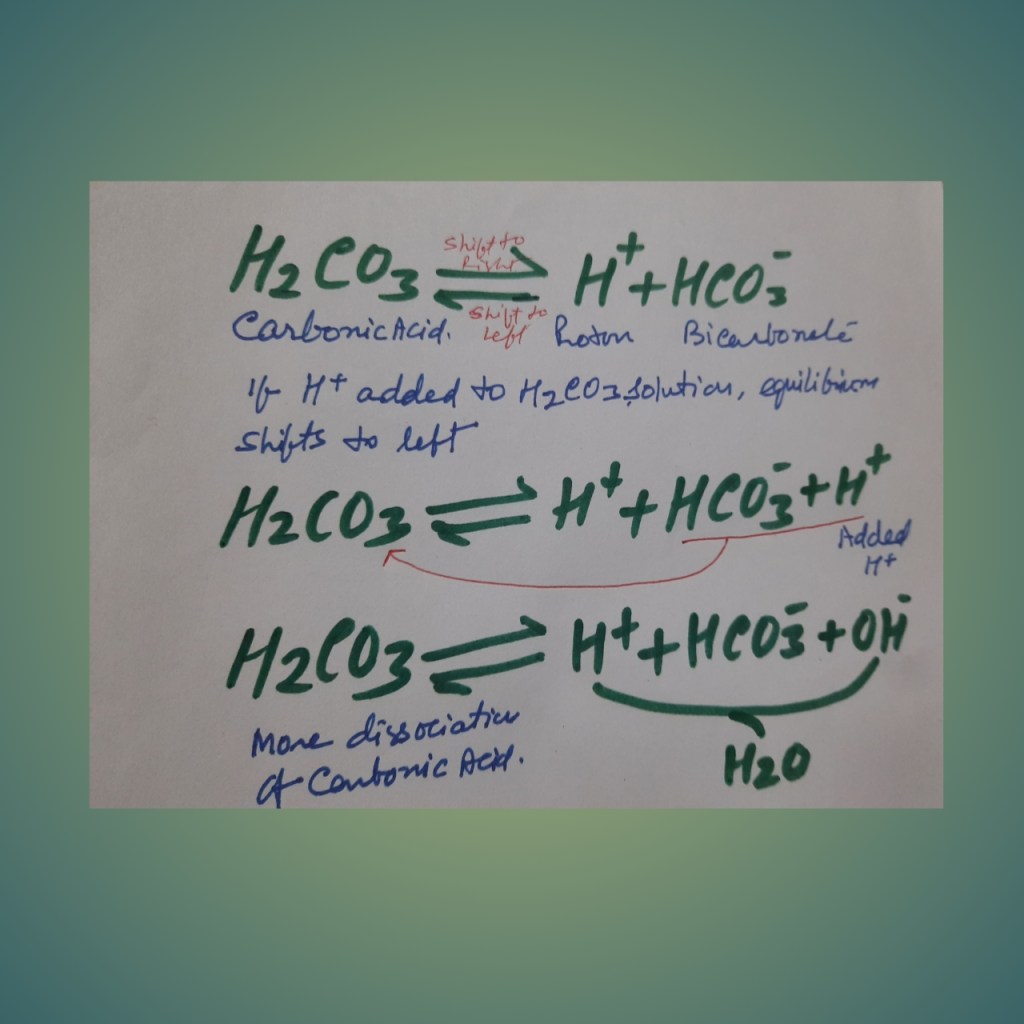
If a powerful acid is added to the solution with the bicarbonate buffer system, more hydrogen ions are added, then the equilibrium shifts to the left. Most of the added hydrogen is removed from the solution maintaining the Ph.
If a base (hydroxyl ion) is added, hydrogen and hydroxyl ions will form water and remove the hydrogen ion from the solution, that decrease [H + ]. As a result, more carbonic acid dissociates, and the decline in hydrogen ion concentration is minimized.
The carbonic acid bicarbonate system is a very effective buffer system in the body due to the:
1. The bicarbonate level in plasma is in equilibrium with the dissolved carbon dioxide controlled by respiration.
2. The Kidneys regulate the plasma concentration of bicarbonate.
The bicarbonate buffer system is rapid as it depends on respiration.
Hemoglobin – The hemoglobin buffer system is responsible for 90% of the buffering capacity for carbonic acid. Red blood cells contain hemoglobin.
The buffering action of hemoglobin is due to imidazole groups of the histidine residues-38 histidines in one hemoglobin molecule.
Deoxy hemoglobin or reduced hemoglobin is a more effective buffer.
Hydrogen ions produced by CO2+H 2O àH 2CO3+ H+ are buffered by hemoglobin, which is reduced by oxygen dissociation.
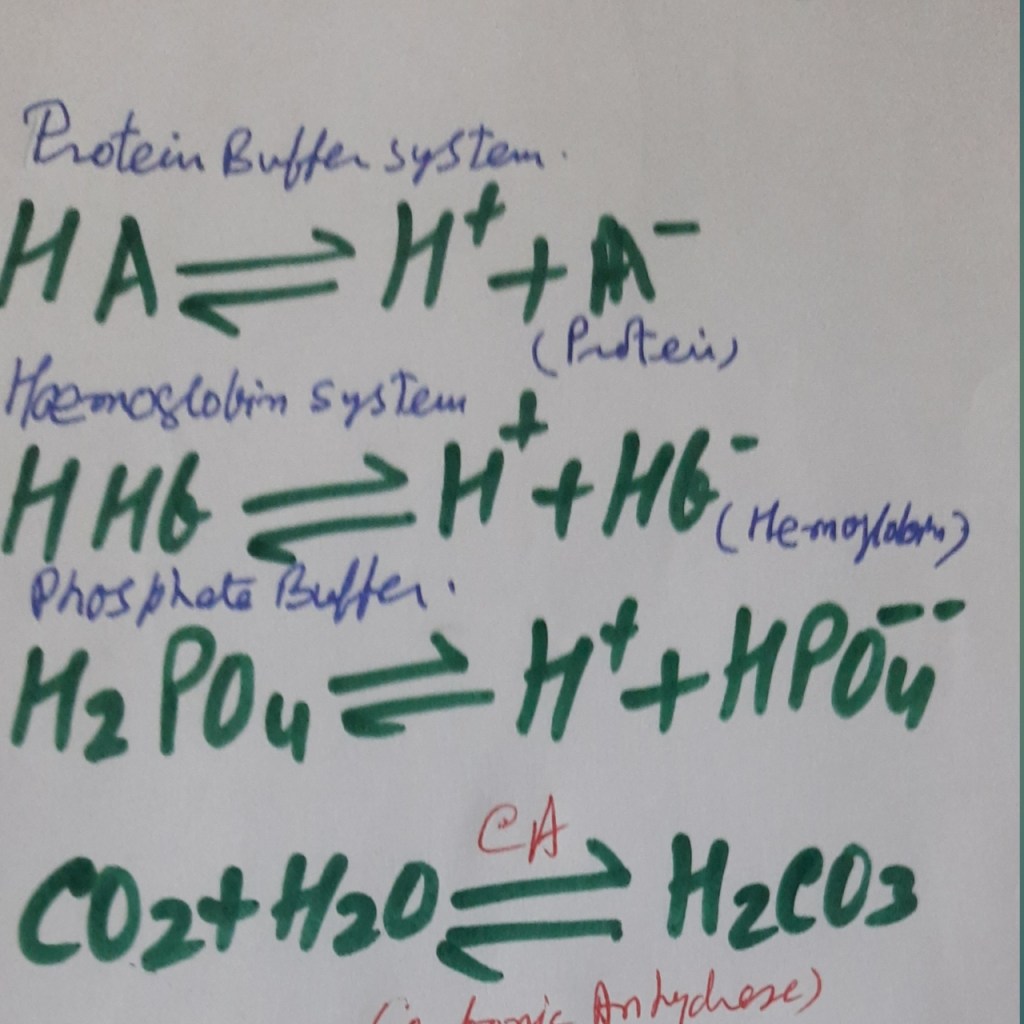
Protein buffer systems-.
Proteins are amphoteric -behave as acids or bases according to the body’s conditions. At average pH (7.4) plasma proteins are ionized-
C terminal end is the form of COO–, will bind with any added acid while
The N-terminal end is as NH3 + that binds with any added alkali.
All proteins are buffers and function mainly intracellular. Therefore, it is responsible for all intracellularly buffering action and about 2/3 of the buffering power of the blood.
Amino acids form proteins. They contain positively charged amino groups and negatively charged carboxyl groups.
The charged groups bind hydrogen and hydroxyl ions and thus work as buffers.
Phosphate buffer system:
The phosphate concentration is low in plasma, so their buffering power is less. But phosphate compounds are abundant in the intracellular fluid, therefore an important intracellularly.
When the carbonic anhydrase enzyme is absent (for example, there is no carbonic anhydrase in plasma.) reaction of carbon dioxide and water to form bicarbonate proceeds slowly in either direction.
When the enzyme is present (an abundant amount in RBC, Gastric acid secretion cells, and Renal tubular cells), the reaction of carbon dioxide and water forms bicarbonate which proceeds swiftly in either direction.
Acidosis or acidemia is an abnormal condition in which the acid content in the human body is high. An increase in the hydrogen ion concentration
decrease in pH value.
Acidosis is of two types-
Metabolic acidosis ,and Respiratory acidosis.
Alkalosis or alkalemia is a clinical condition due to excessive accumulation of base or loss of acid, sufficient to increase pH level above normal.
Types of alkalosis:
Metabolic alkalosis and Respiratory alkalosis.
Acidosis is due to
a] increase in hydrogen [acidic compounds-due to1.exogenous-increased intake 2. Endogenous-increased production in the body and reduced elimination.
B] decreased base in the body
Low intake- exogenous,
Decreased formation and increased elimination.
Alkalemia is due to
Increase in the level of base:
1. Exogenous-increased intake 2. Endogenous-increased production in the body and reduced elimination and,
B] Decreased level of acid in the body
Low intake- exogenous,
Decreased formation and increased elimination.
Role of Kidneys
Kidneys will increase the excretion of hydrogen ions and increase the absorption of bicarbonates to maintain body pH. This is a slow process.
Role of the respiratory system